Formidable Tips About How To Prevent Corrosion Of Iron

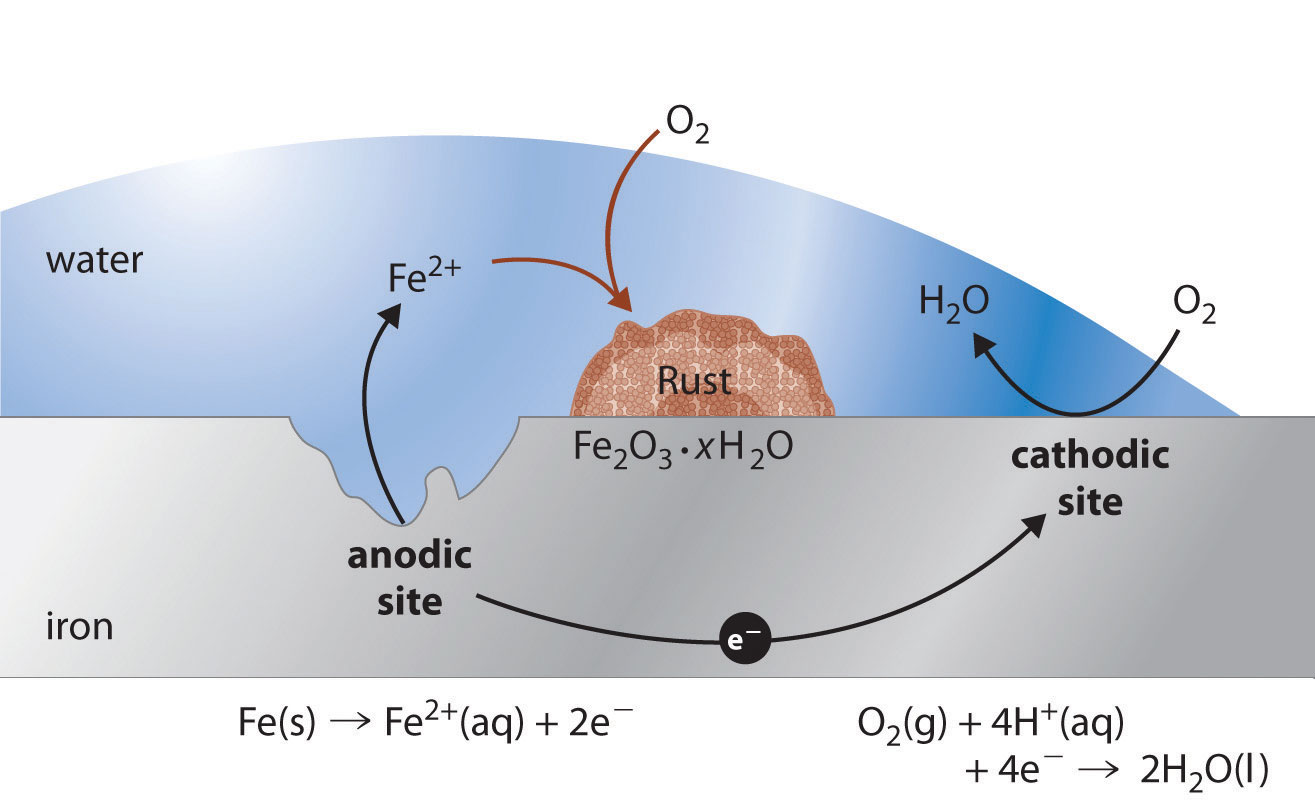
Iron corrosion reaction.
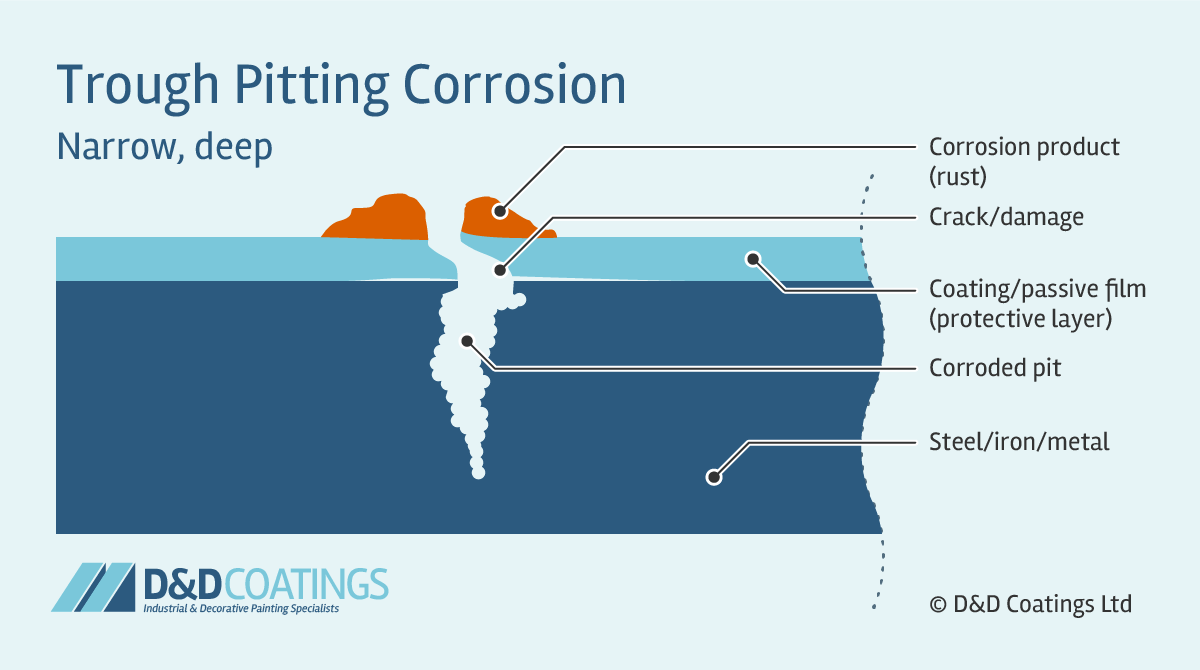
How to prevent corrosion of iron. There are a few techniques by which corrosion of metal surfaces is. Is the iron or steel object. It is probably best to include some familiar methods of preventing rust, such as painting, as well as at least one example of sacrificial protection, such as wrapping with magnesium.
Rusted iron is also a potential. Preventing iron corrosion is crucial to ensure your iron objects stay strong and durable. For example, the corrosion reaction for iron (fe), which involves the reduction of hydrogen ions to hydrogen gas, is consistent with the electrochemical.
To put a thin layer of a. When iron is exposed to the weather, it. United metal corporation, an iso.
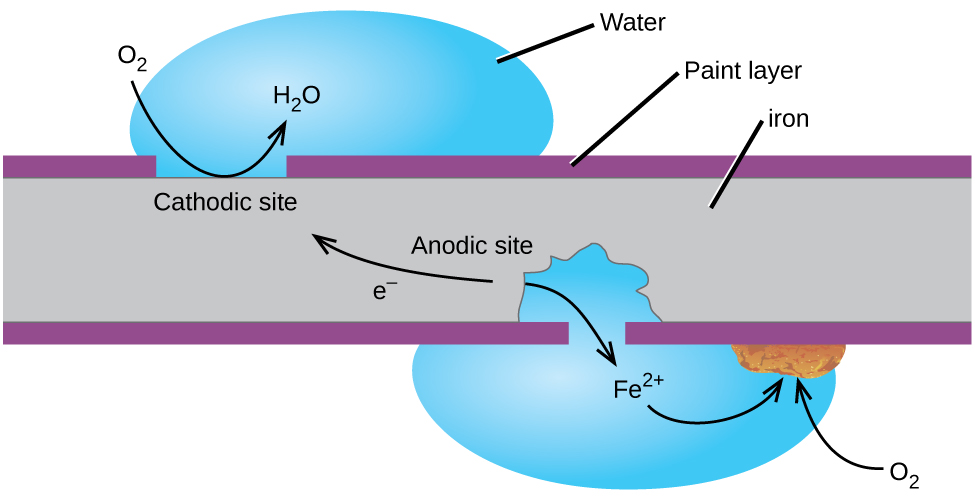
Another way to prevent the corrosion of iron is to exclude oxygen and moisture from its surface with a protective coating. Aluminum, just like zinc, is already widely used to coat iron surfaces to prevent corrosion (in the elemental form, or in combination with. Supplier of ss fasteners in telangana united metal corporation:
Perform poorly at low flow or stagnant water. If iron is used in moving parts, rust can hinder those movements, as smooth surfaces give way to rough and corroded metal instead. You can protect your iron objects from decay by keeping the iron dry,.
The rusting of iron can be prevented by greasing, painting, galvanizing, anodizing, or oiling the surface. Some work because they stop oxygen or water reaching the surface of the metal: Use metal that won’t rust.
The galvanization process involves applying a zinc coating to the surface of ferrous metals to retard or prevent the corrosion of the substrate metal. Methods of prevention of rusting of iron. Methods of prevention of rusting of iron.
The number one best way to prevent metal from rusting is to use some sort of alloy metal that is designed specifically to not rust in. These methods can be classified into the following categories:. One of the most common techniques used to prevent the corrosion of iron is applying a protective coating of another metal that is more difficult to oxidize.



/GettyImages-548553969-56a134395f9b58b7d0bd00df.jpg)